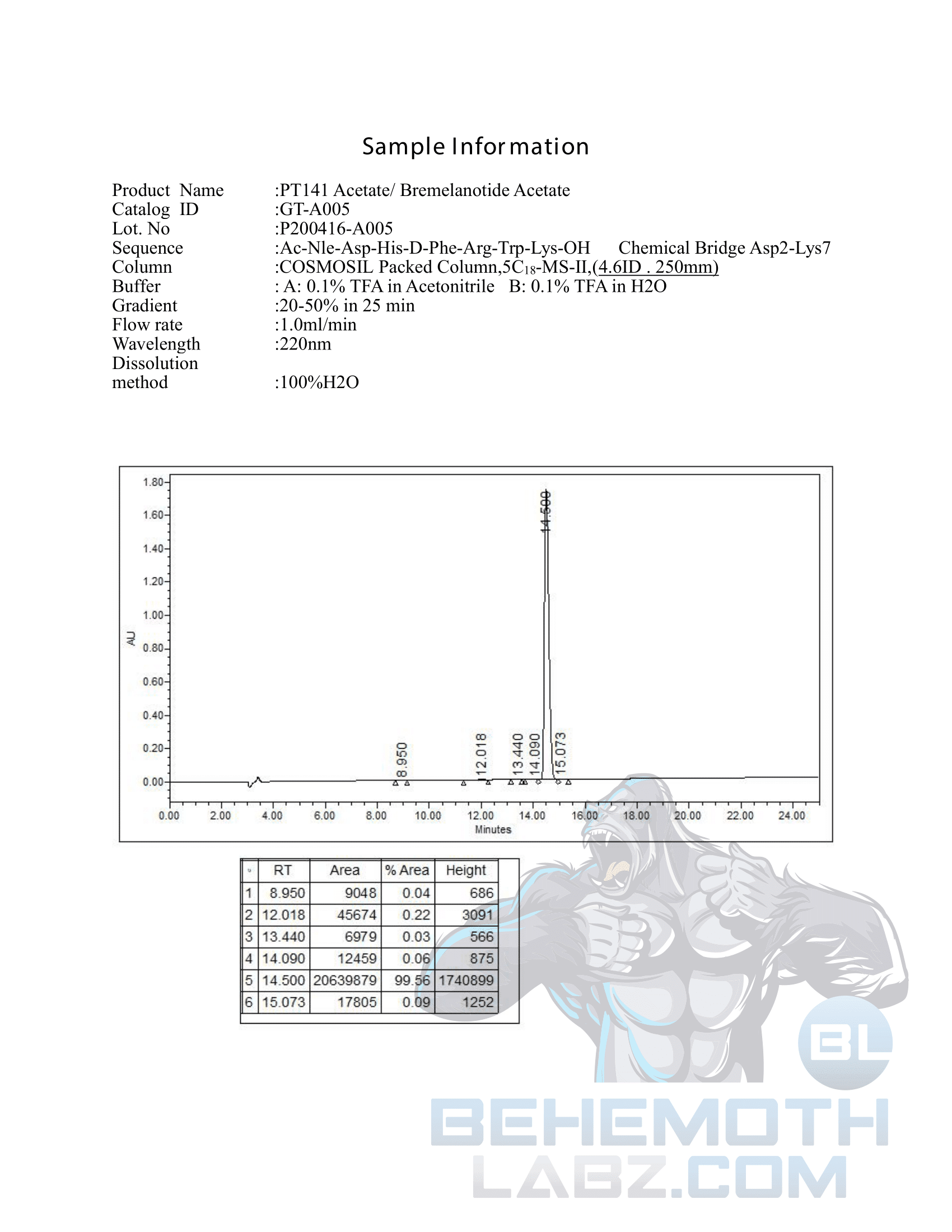
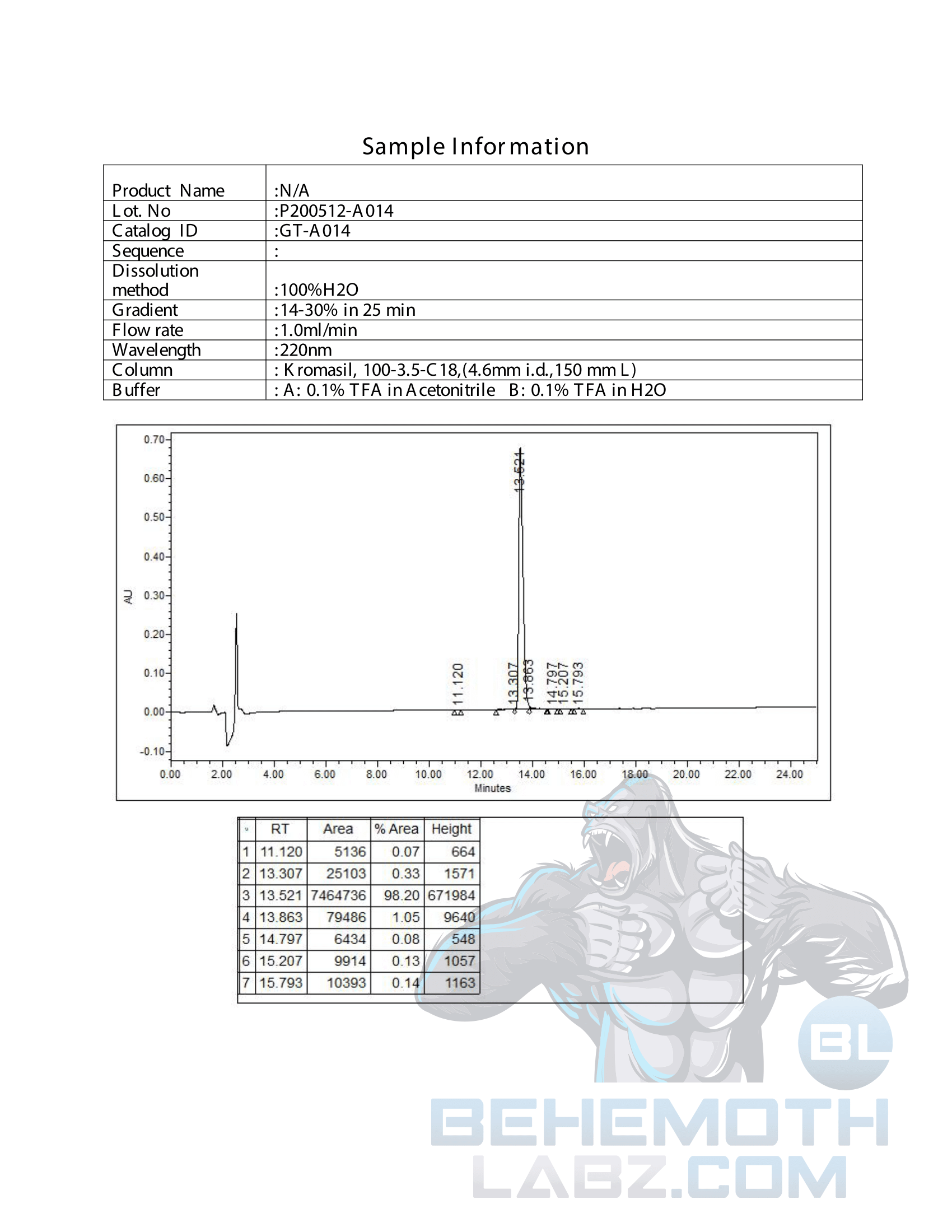
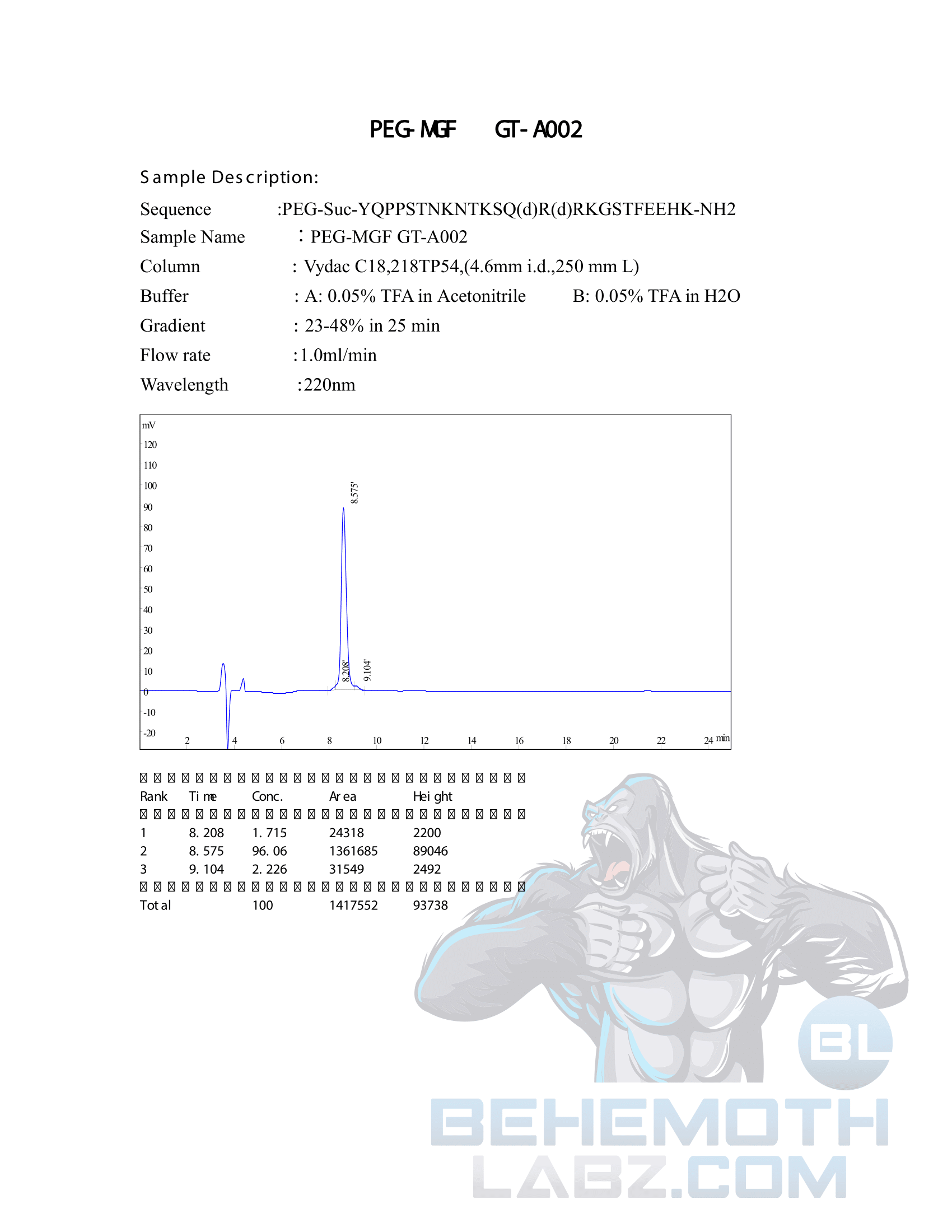
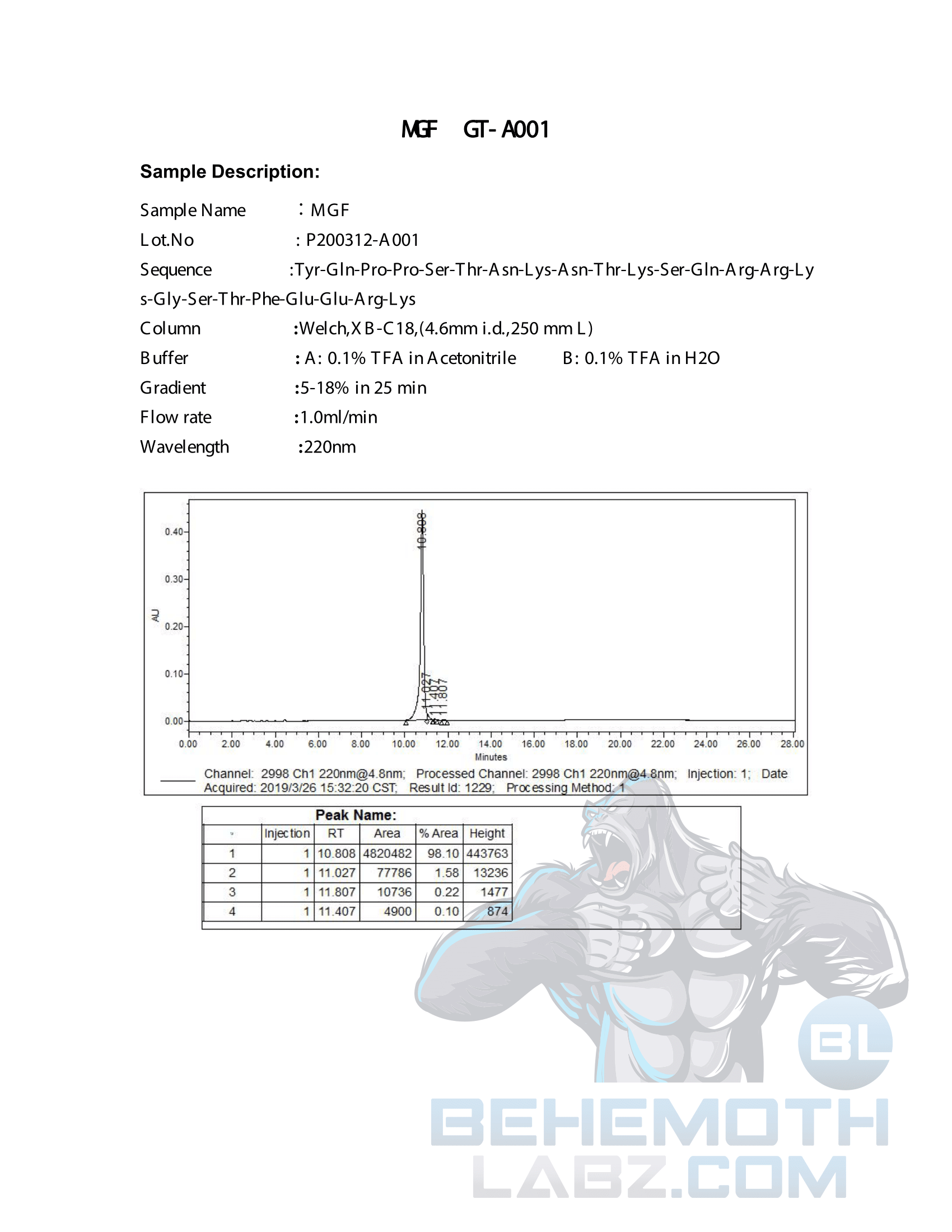
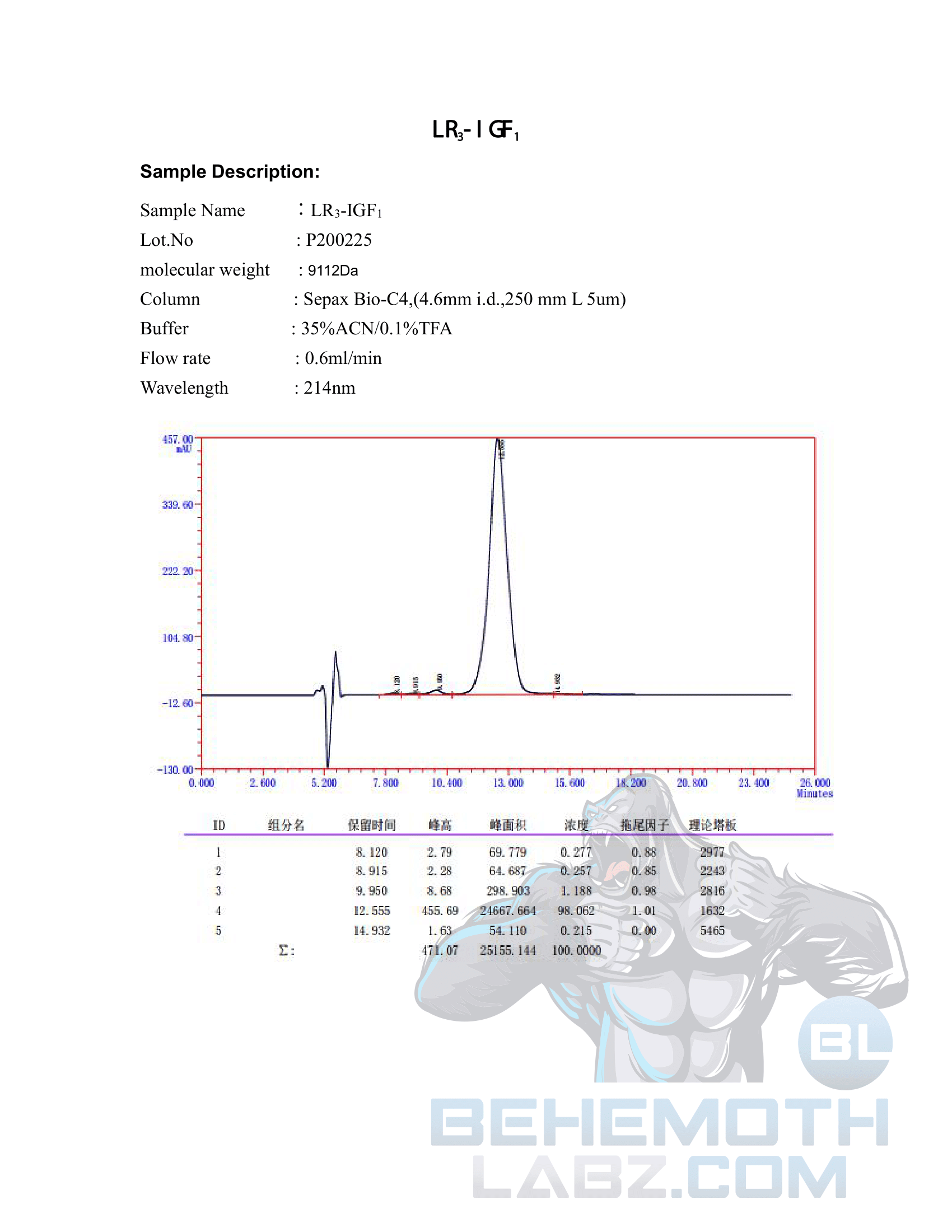
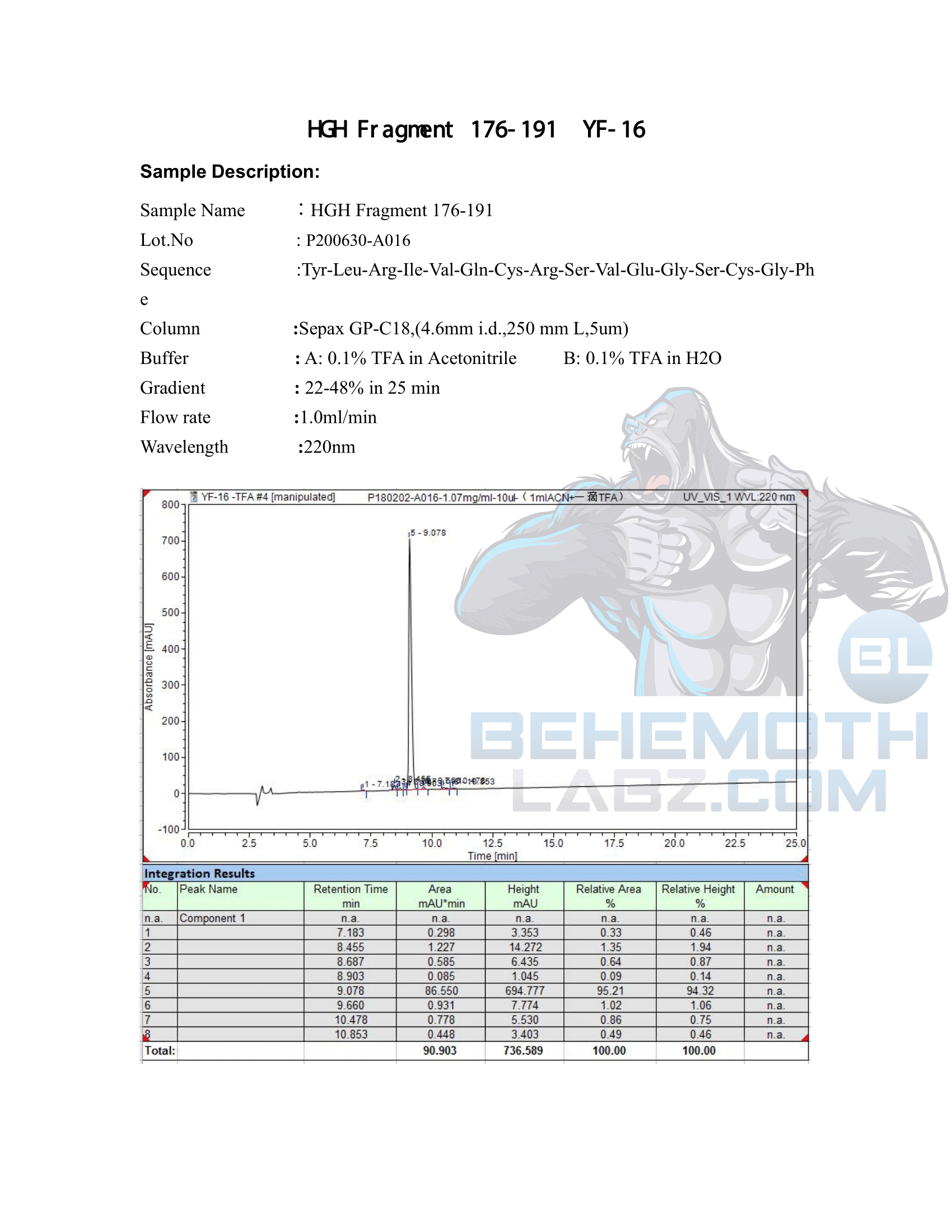
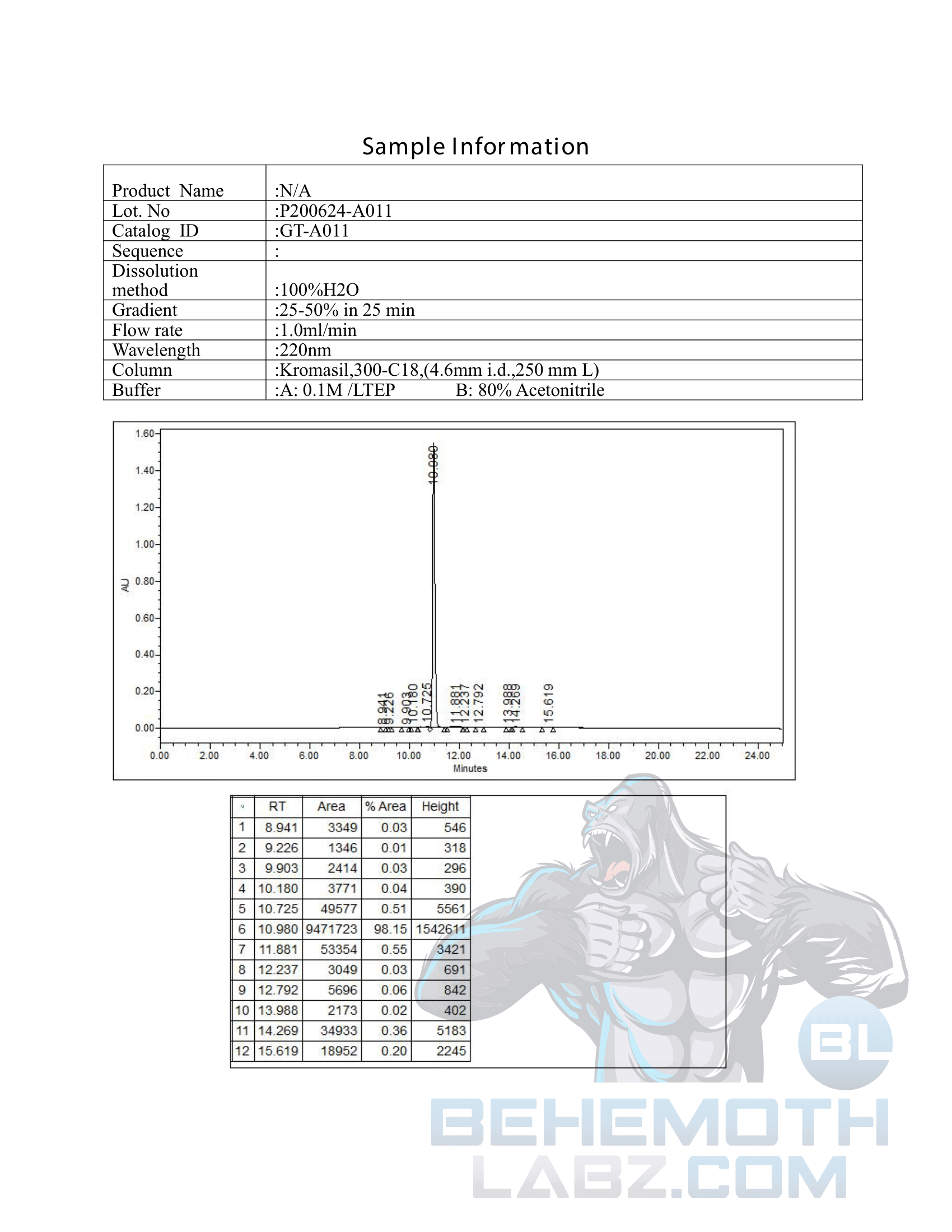
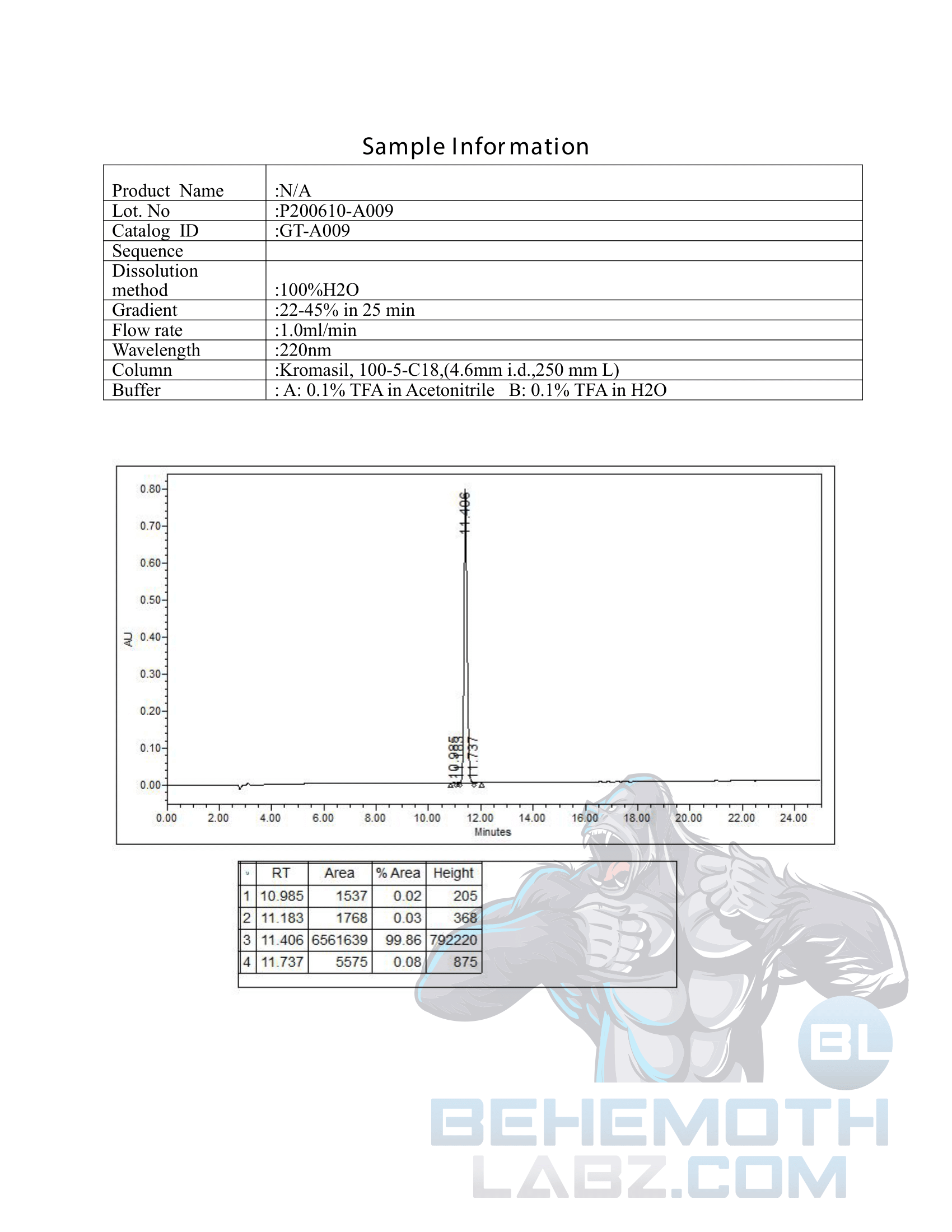
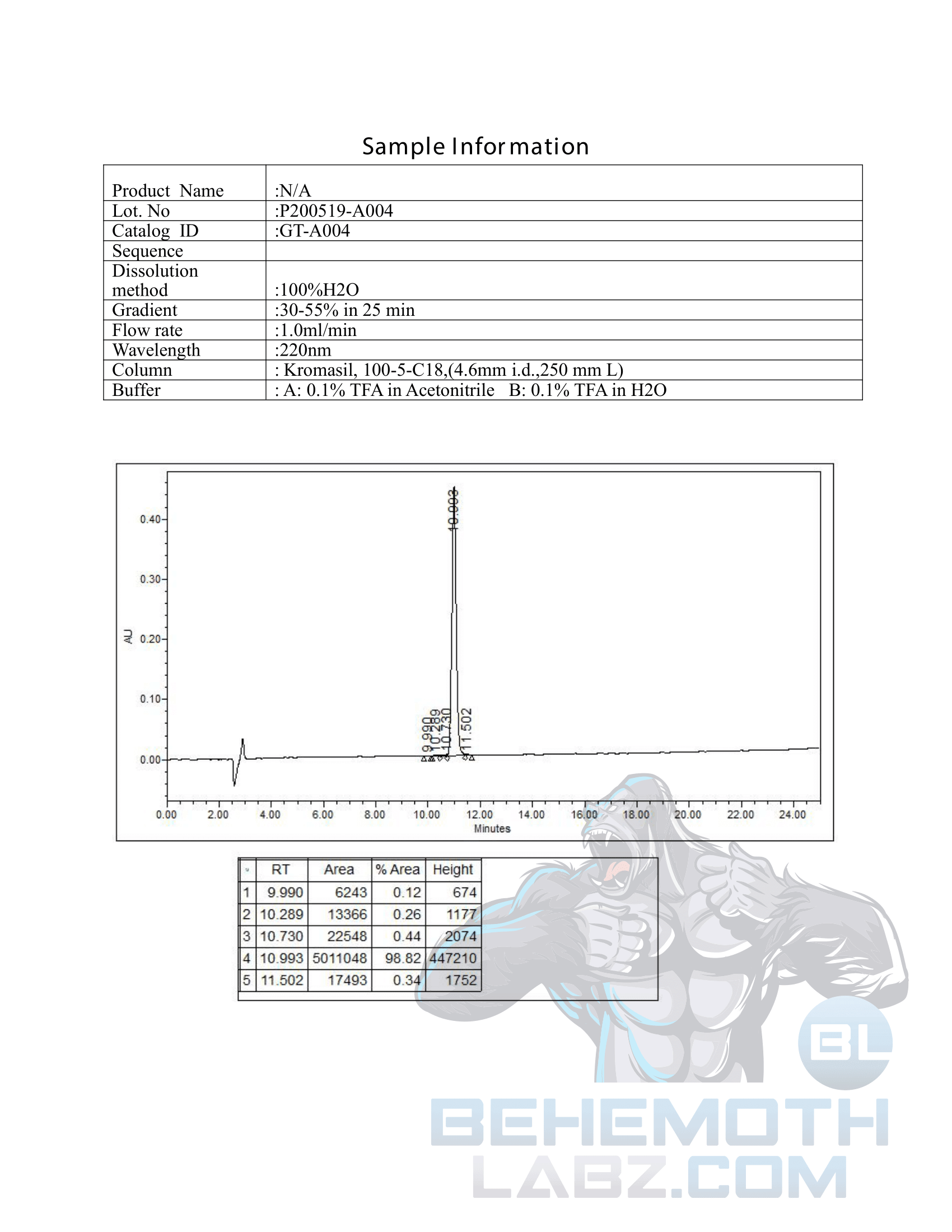
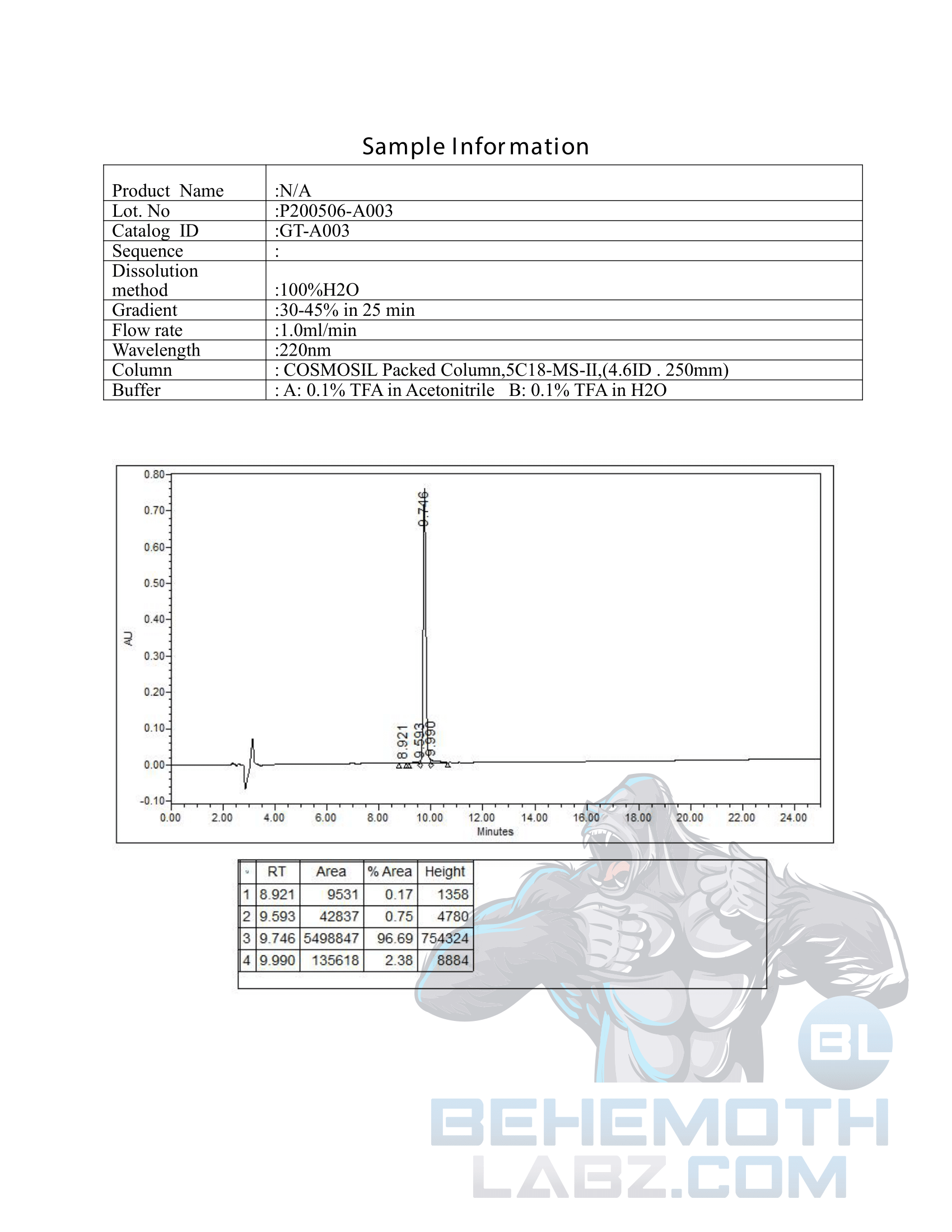
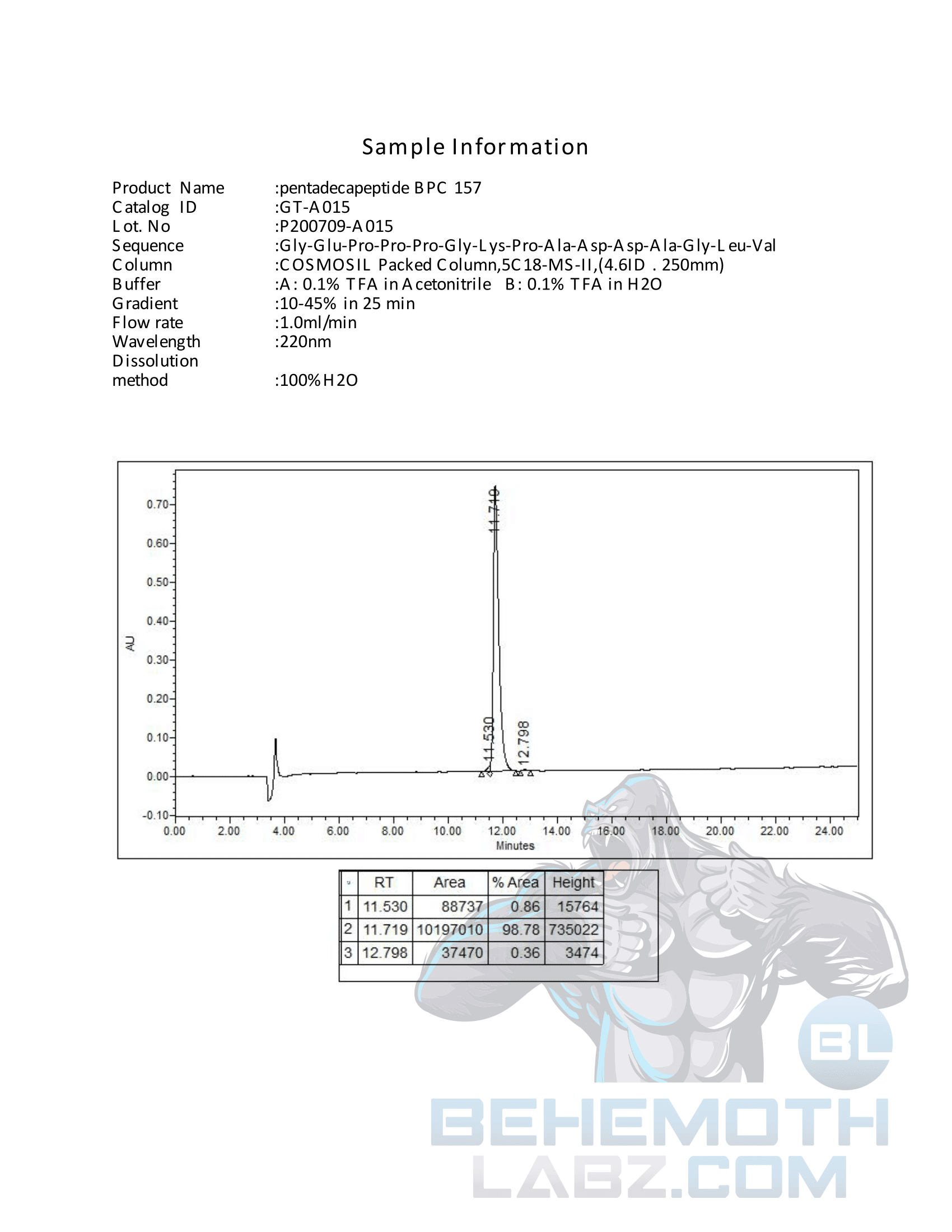
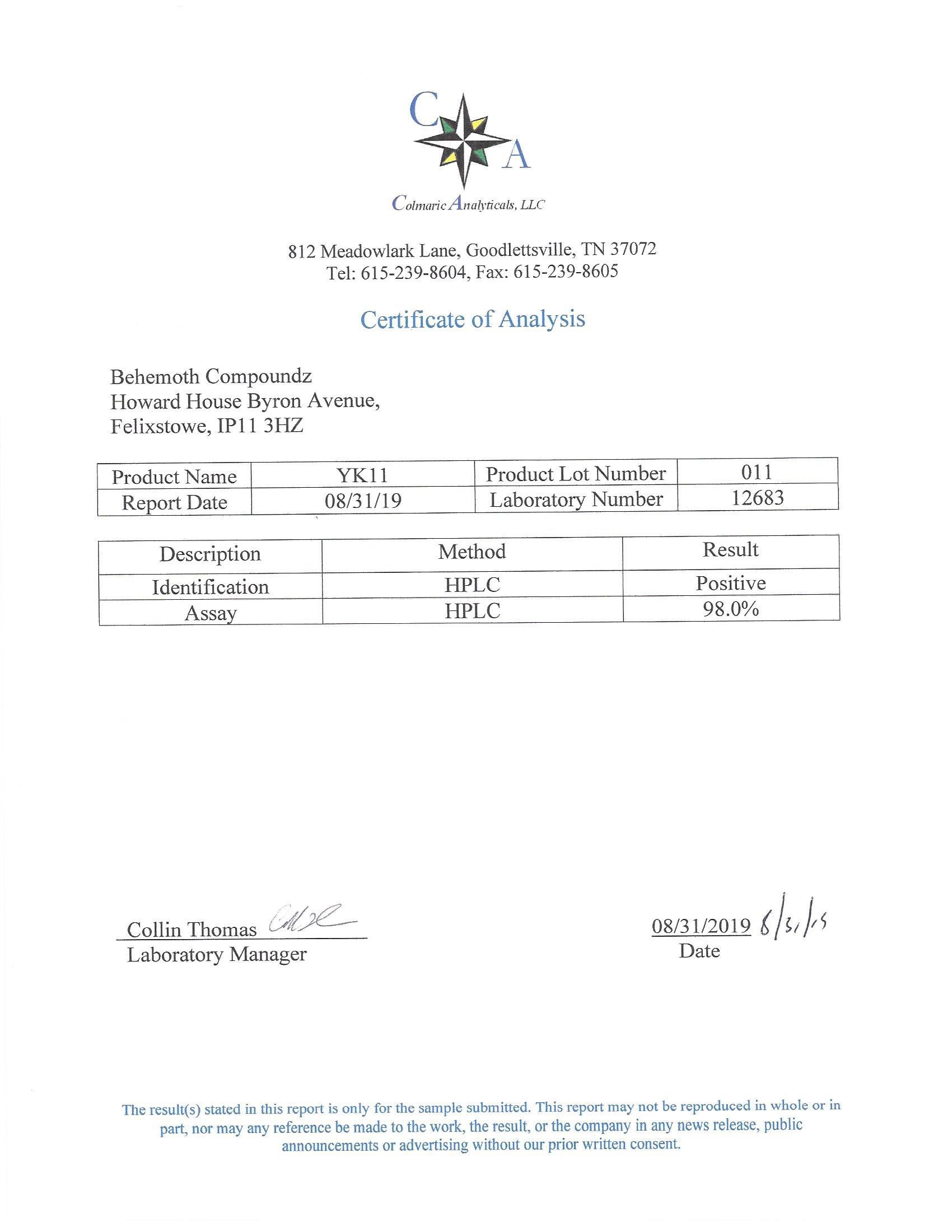
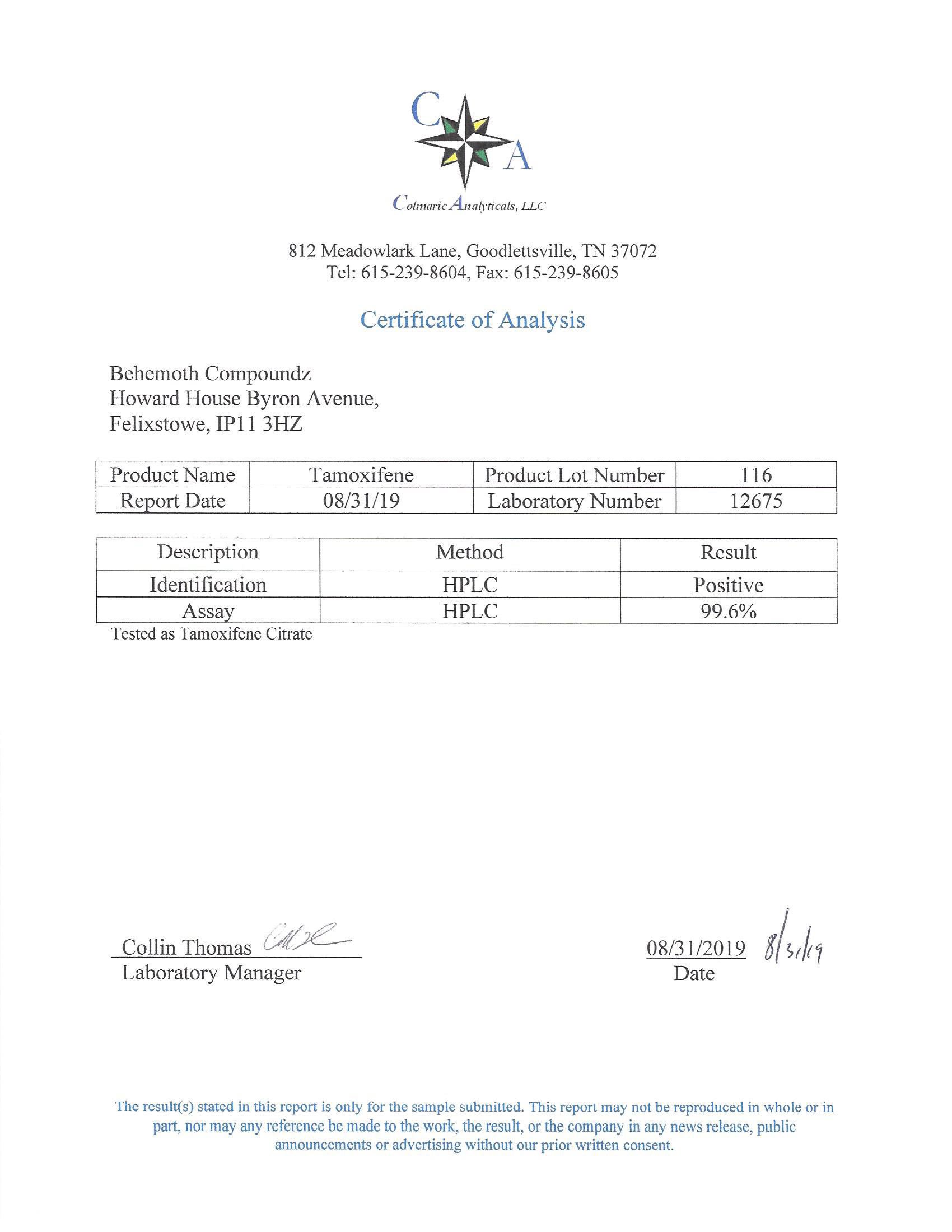
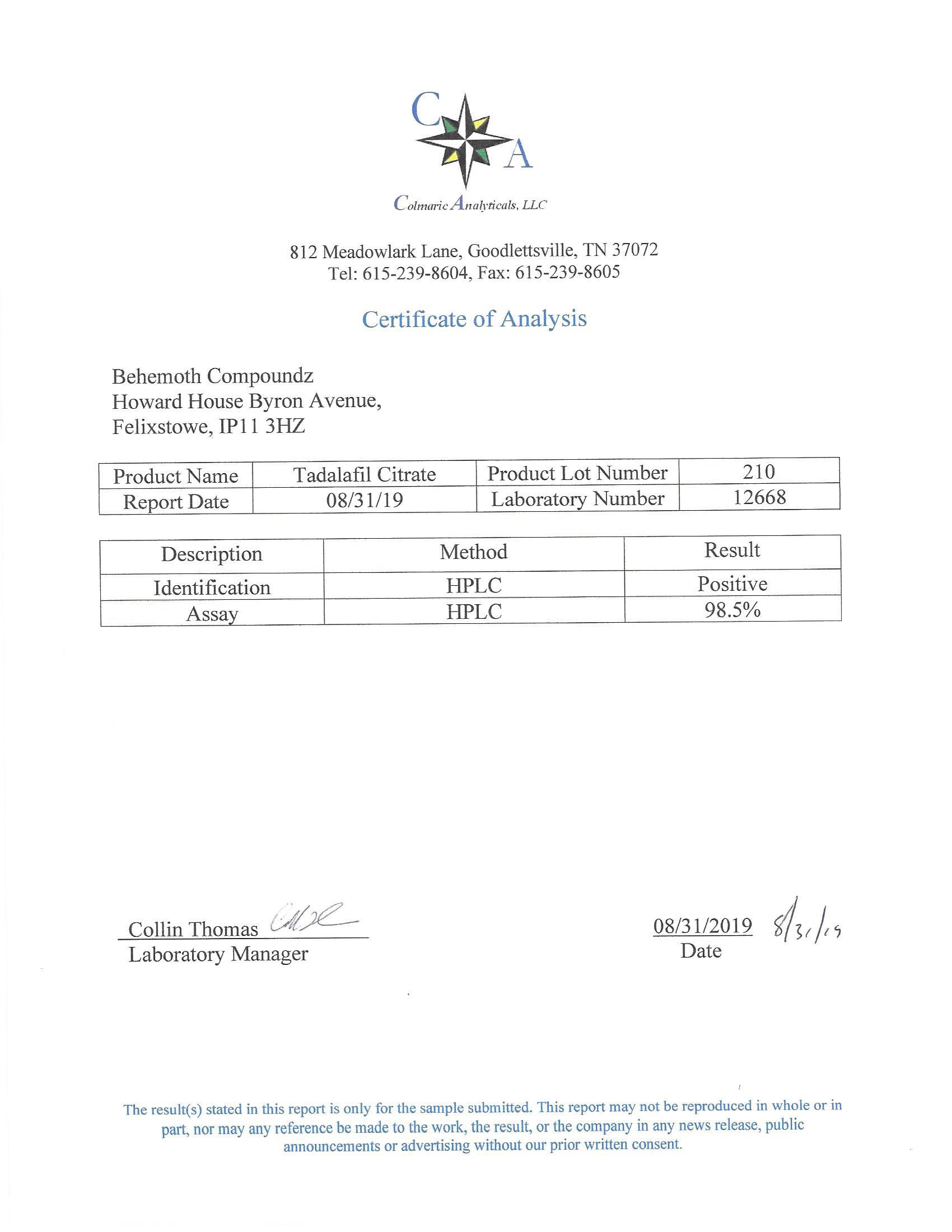
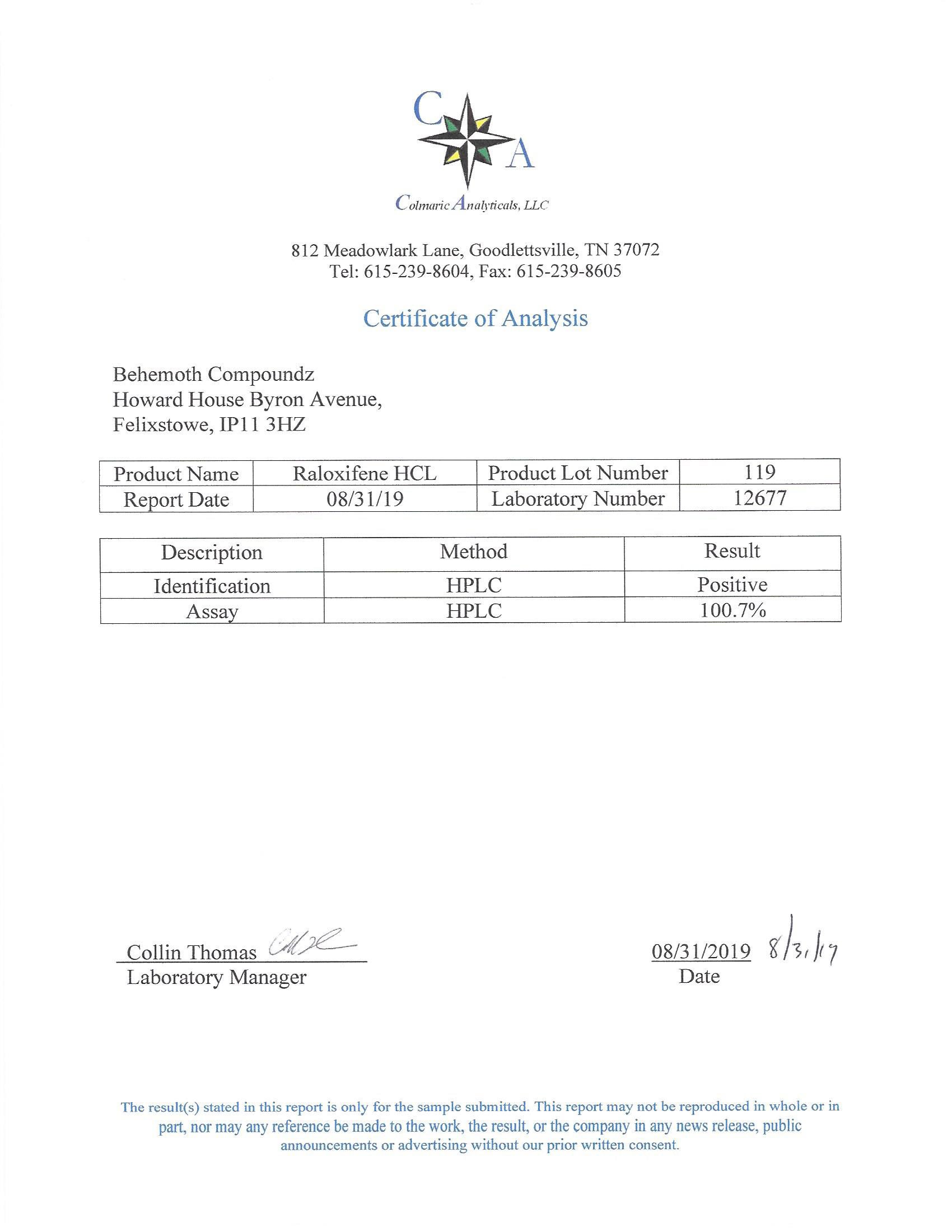
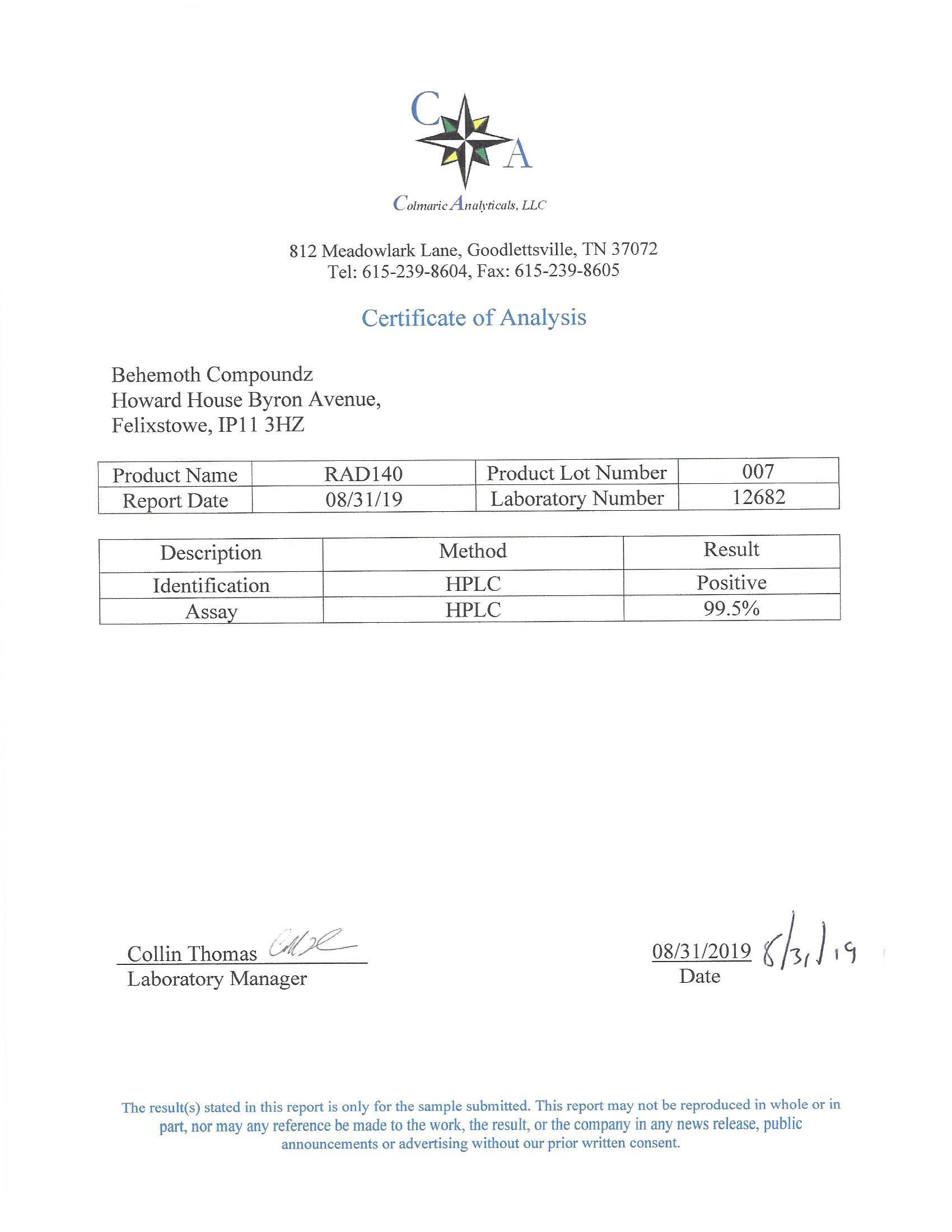
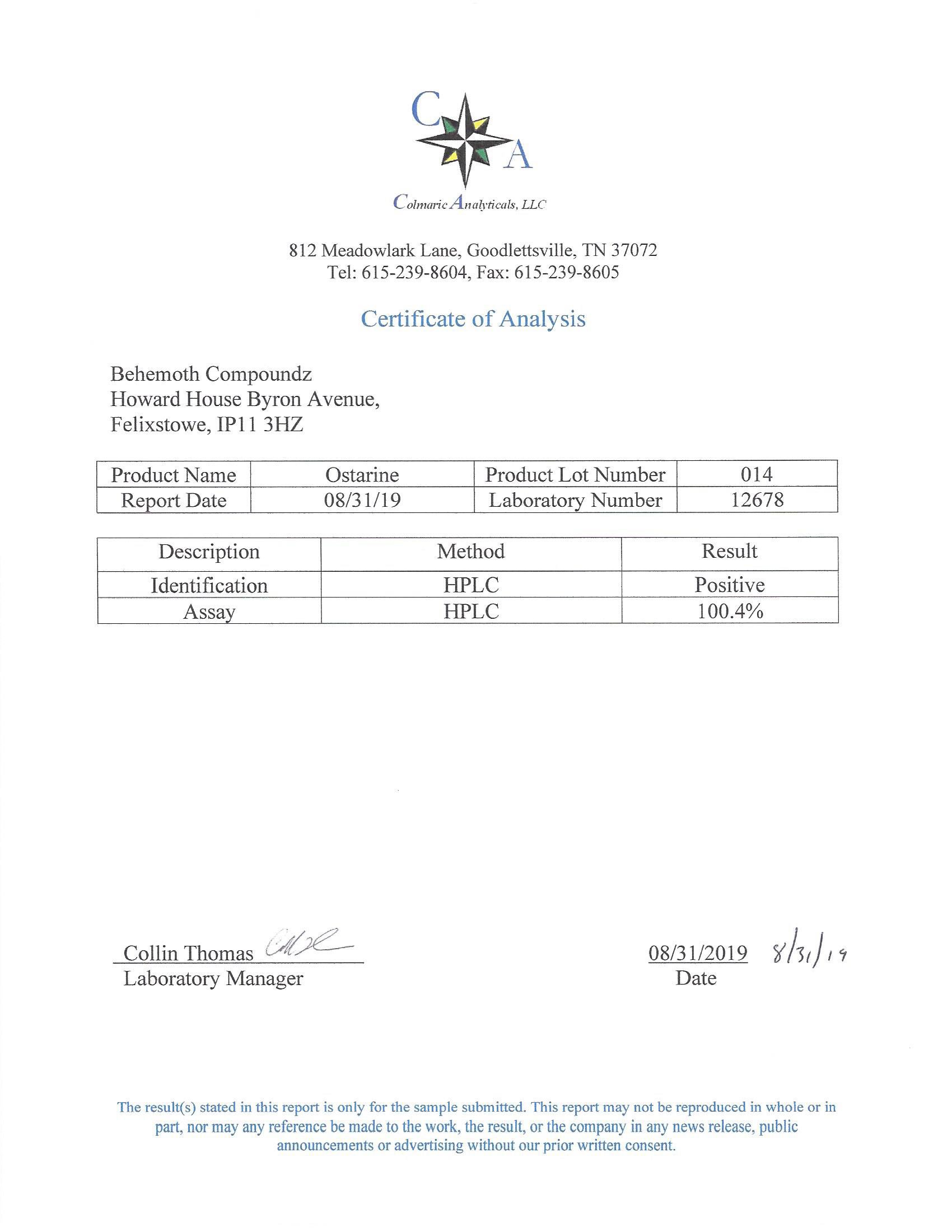
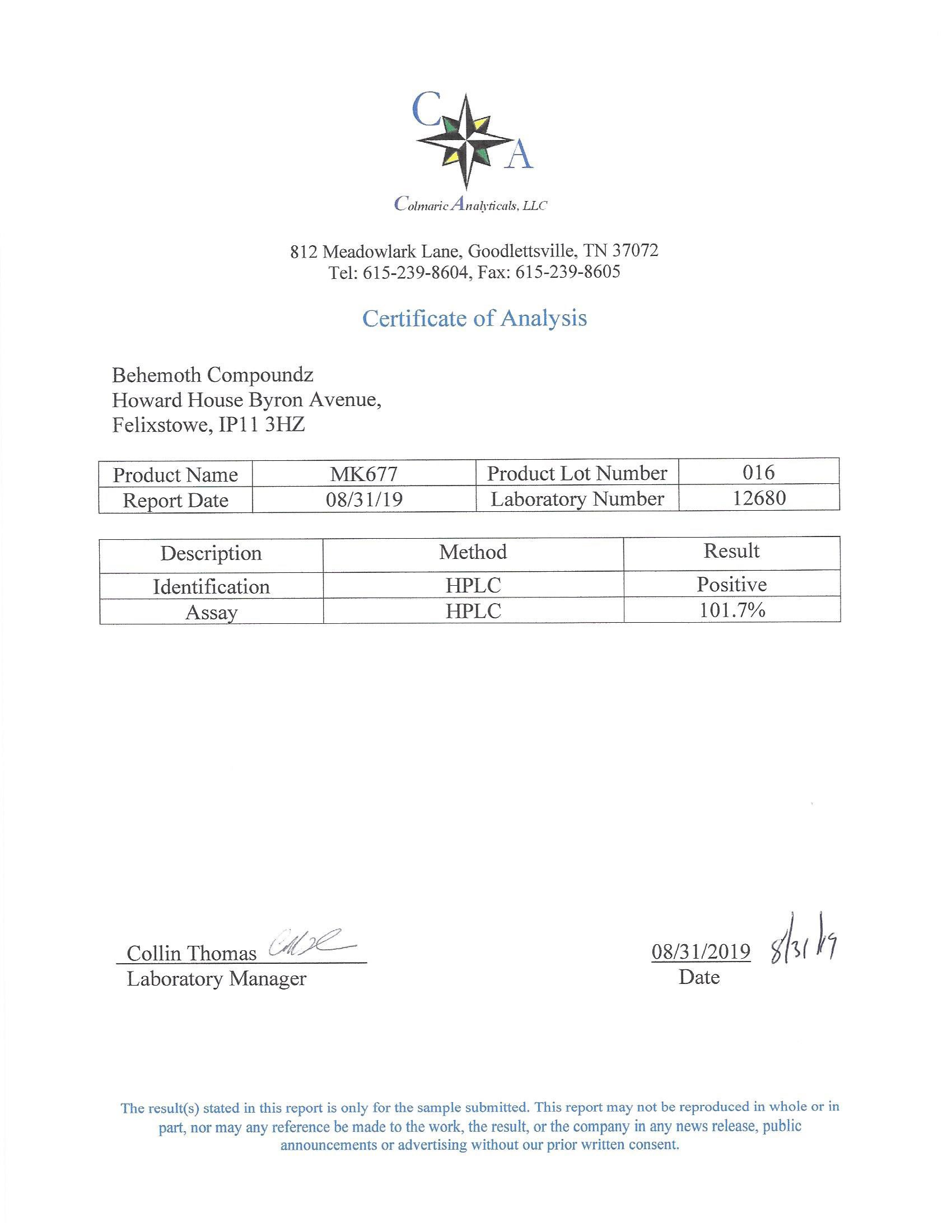
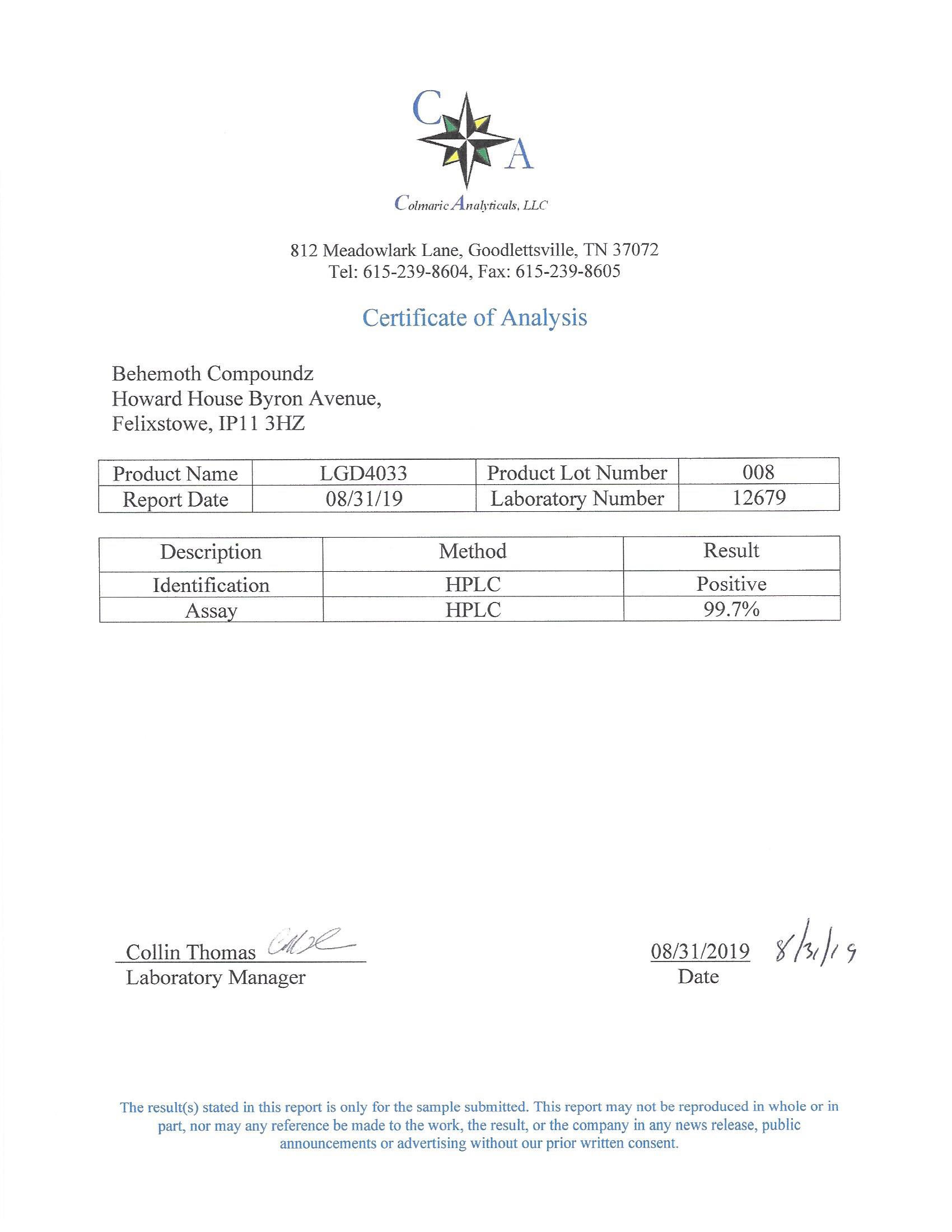
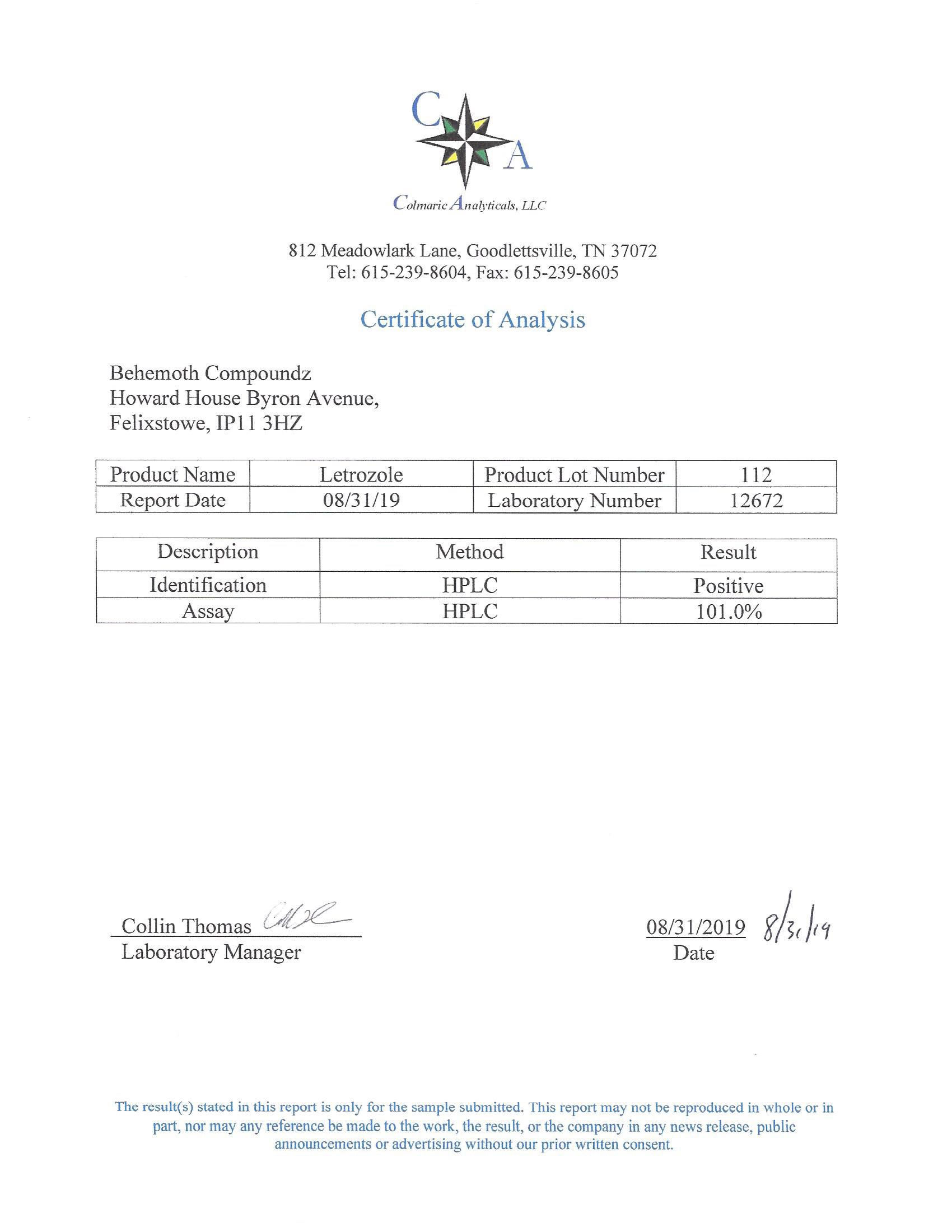
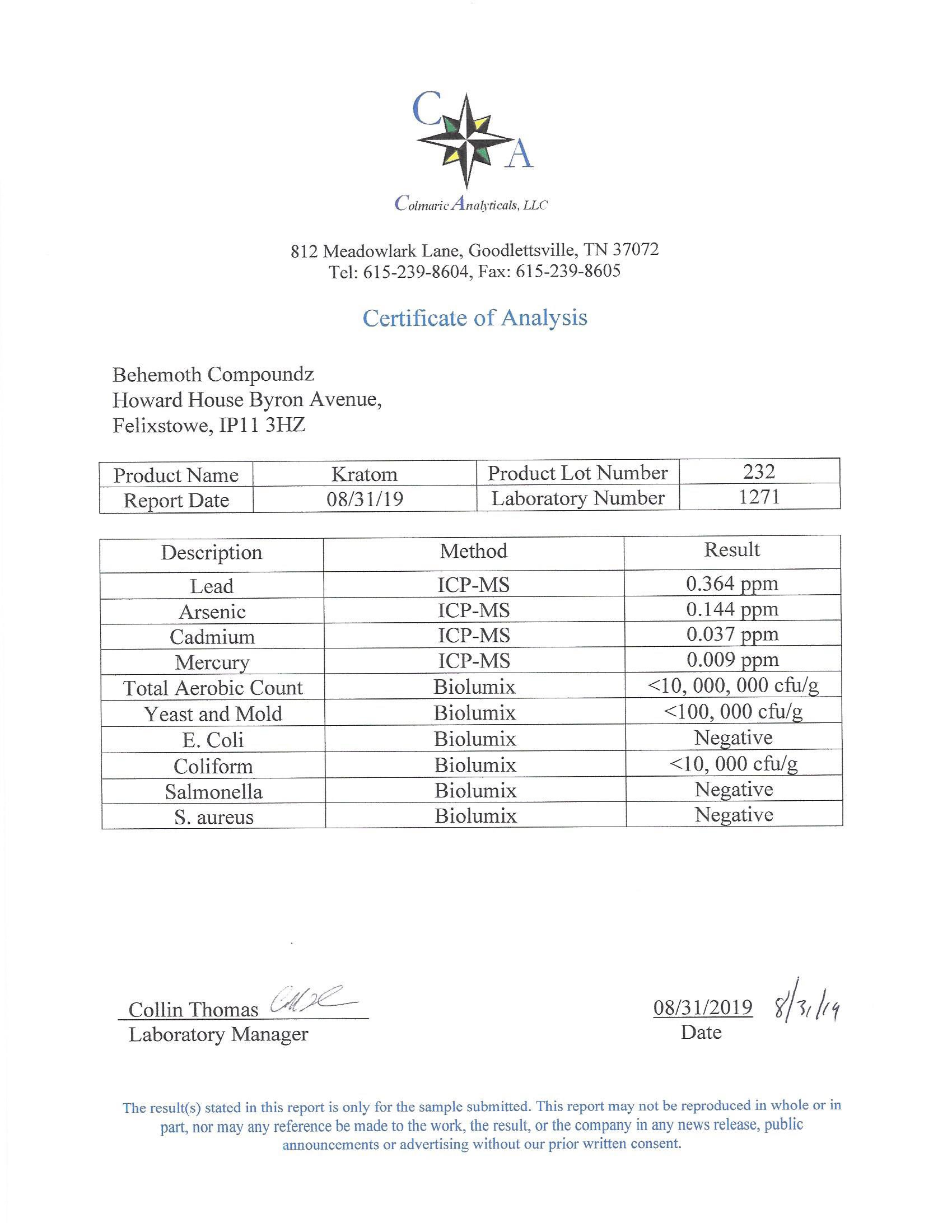
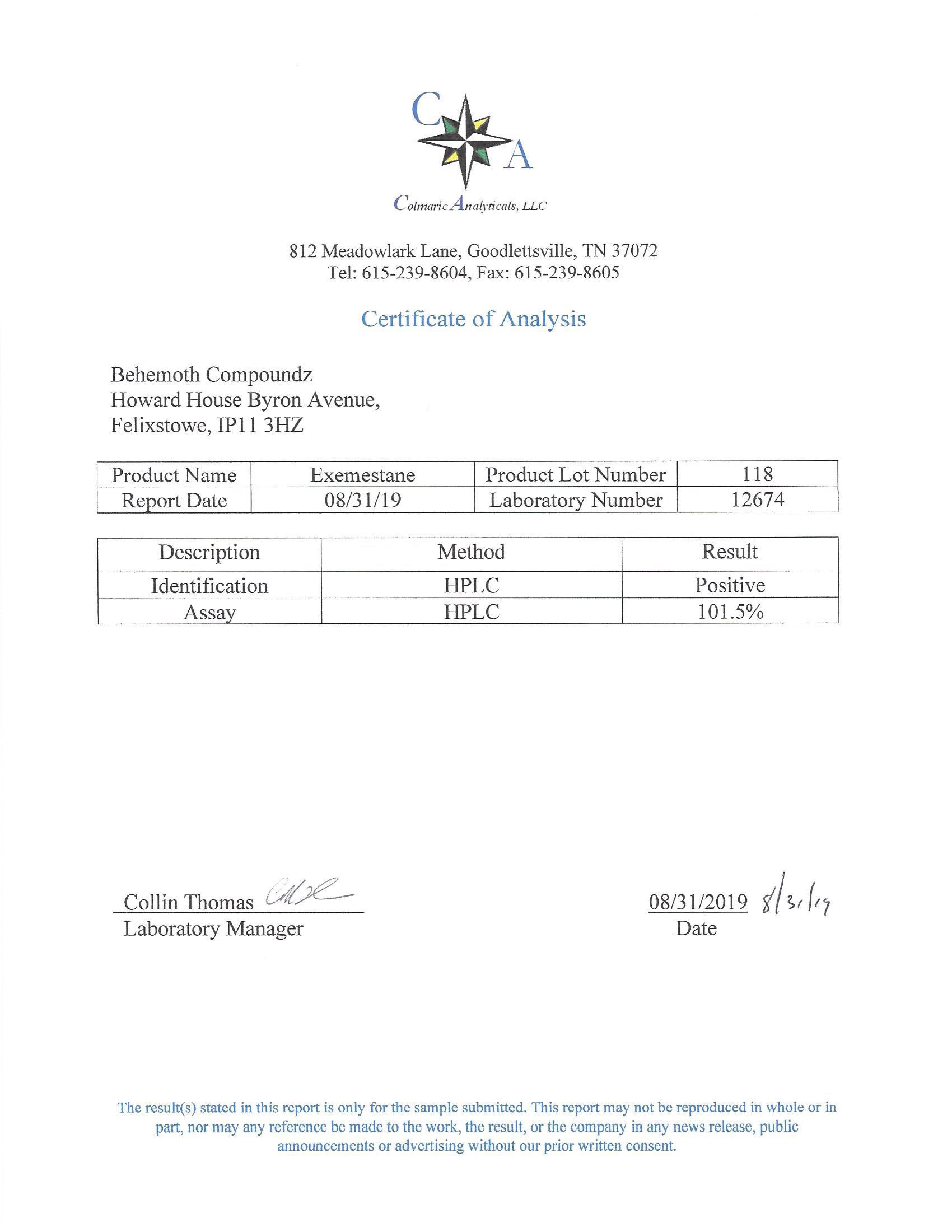
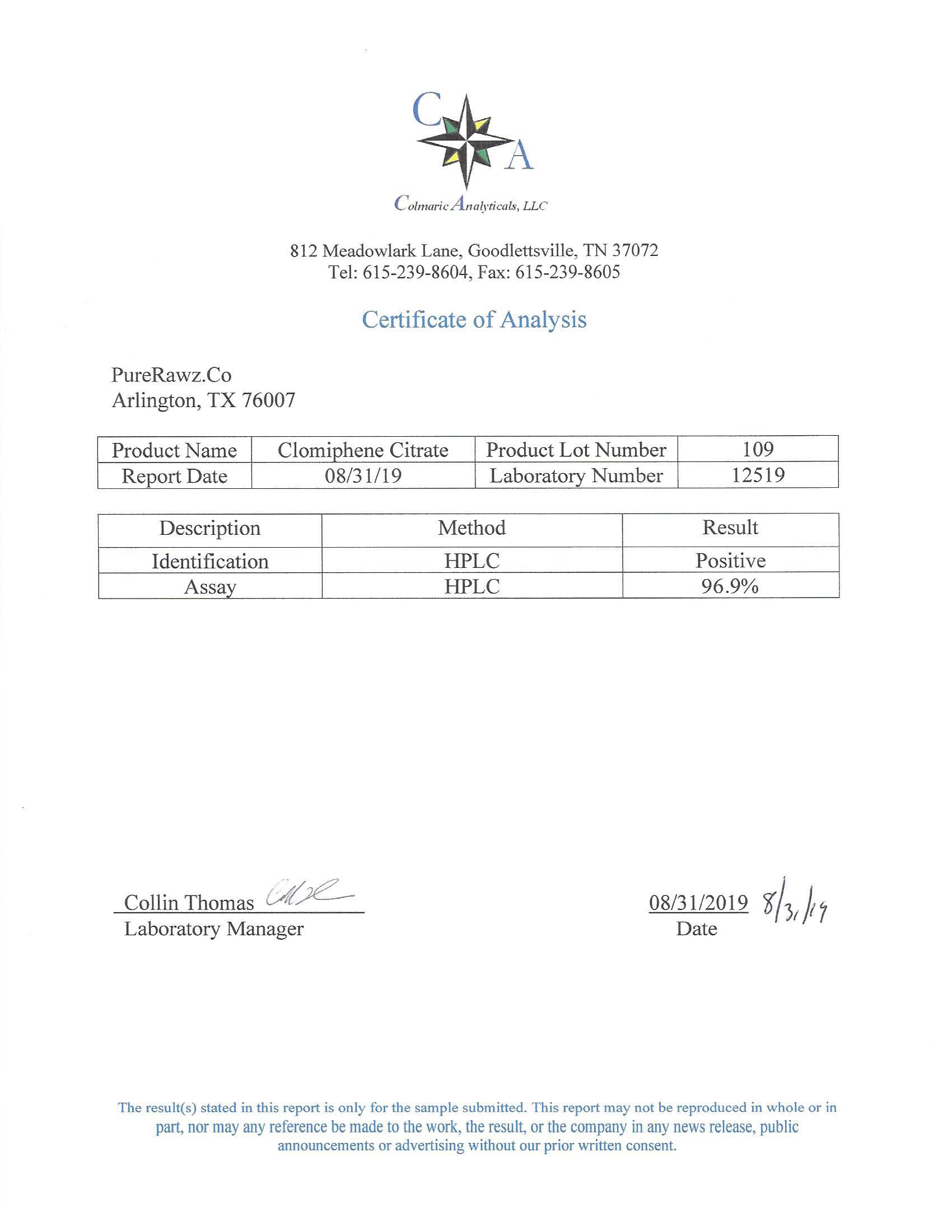
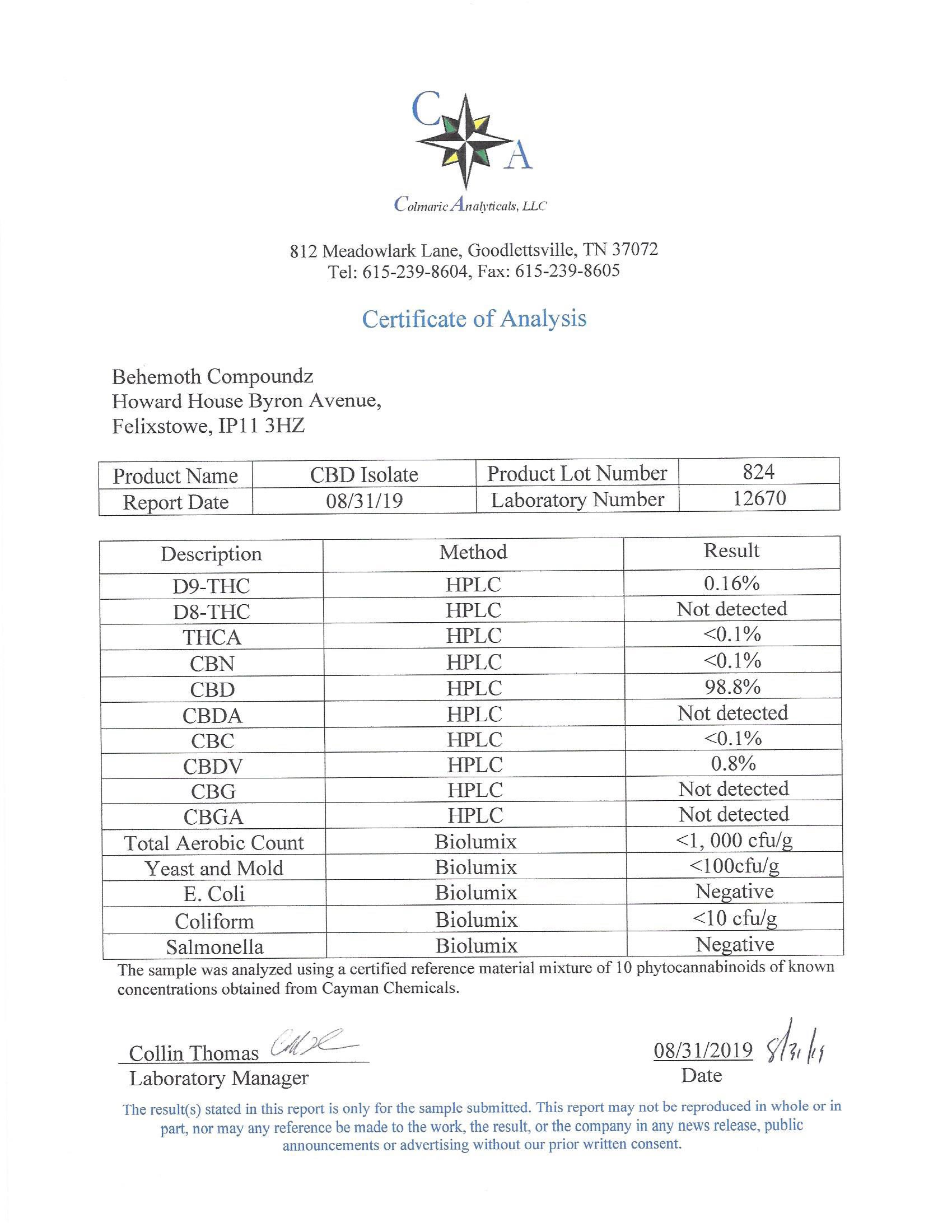
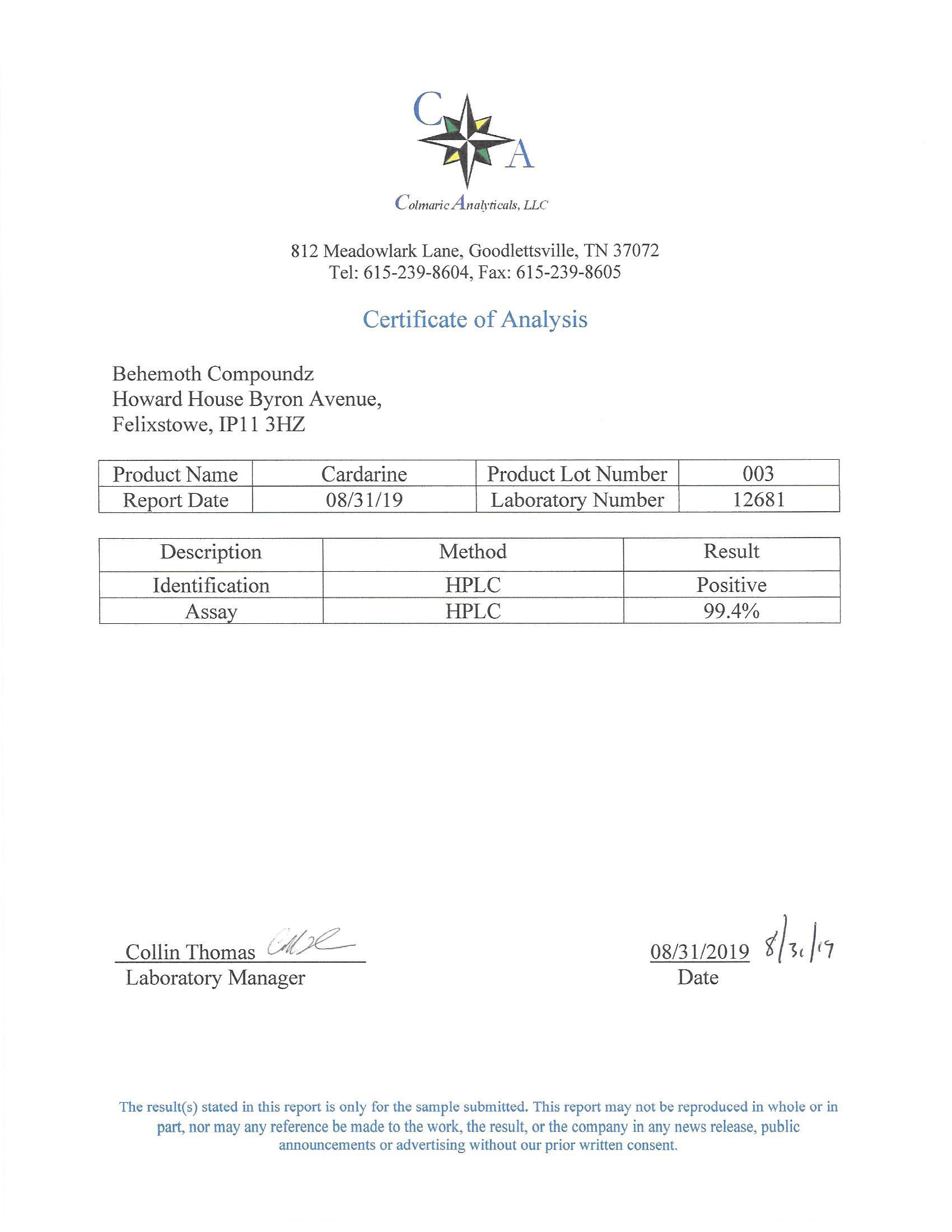
The methods mentioned above cannot or will not work for some products. Alternative techniques must be applied in these circumstances. These techniques can consist of:
Purity Thermogravimetric Analysis
The mass of a sample is measured over time as the temperature changes in the thermogravimetric analysis (TGA), a technique for thermal analysis. Phase changes, absorption, adsorption, and desorption, as well as chemical phenomena like chemisorptions, thermal decomposition, and solid-gas reactions are all revealed by this measurement (e.g., oxidation or reduction).
Identification & Purity Using UV-Vis Spectroscopy
Absorption or reflectance spectroscopy in the ultraviolet-visible spectral range is referred to as ultraviolet-visible spectroscopy (UV-Vis). This indicates that it makes use of light in the visible and nearby UV ranges. An analyte-containing solution is passed through with the aid of a light beam. The amount of light that is absorbed and the composition of the sample are determined by measuring the wavelengths and intensities of the light that passes through.
Identification and purity using FTIR Spectroscopy
Any absorption spectroscopy, including FTIR, UV-Vis, and others, aims to quantify how well a sample absorbs light at various wavelengths. By using the “dispersive spectroscopy” method, the simplest way to accomplish this is to illuminate a sample with a monochromatic light beam, measure how much of the light is absorbed, and repeat for each different wavelength.
In order to determine how a solid, liquid, or gas absorbs or emits infrared light, one can use Fourier-transform infrared spectroscopy (FTIR). High-spectral-resolution data are simultaneously gathered over a broad spectral range by an FTIR spectrometer. This is a significant advancement over dispersive spectrometers, which can only simultaneously measure intensity over a limited spectrum of wavelengths.
Because a Fourier transform, a mathematical procedure, is used to transform raw data into the actual spectrum, infrared spectroscopy is known as “Fourier-transform” spectroscopy.
Simply put, the chemist believes that a sign of composition is the physical change that takes place when a known reagent is added to a solution that already contains the analyte.
 Greenz Bundle
1 × $48.48
Greenz Bundle
1 × $48.48